Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Haruo
Proceedings of International Workshop on Mobile Fission and Activation Products in Nuclear Waste Disposal, p.157 - 171, 2009/05
This study was performed to investigate whether anions diffuse in the interlayer of smectite and to obtain activation energies ( E
E ) of diffusion for ions in the interlayer. The apparent diffusivities for I
) of diffusion for ions in the interlayer. The apparent diffusivities for I and Cs
and Cs ions in compacted smectite with an interlayer space of only 2 water layers were measured as a function of temperature. Purified Na-smectite of which all interlayer cations were exchanged with Na
ions in compacted smectite with an interlayer space of only 2 water layers were measured as a function of temperature. Purified Na-smectite of which all interlayer cations were exchanged with Na ions, was used in the study. In-diffusion experiments for I
ions, was used in the study. In-diffusion experiments for I and Cs
and Cs ions were carried out under the conditions that interlayer space and orientation of smectite stacks were controlled. Dry density in these conditions was 1.79 Mg/m
ions were carried out under the conditions that interlayer space and orientation of smectite stacks were controlled. Dry density in these conditions was 1.79 Mg/m . Basal spacing was also measured by X-ray diffractometry. All diffraction peaks to d
. Basal spacing was also measured by X-ray diffractometry. All diffraction peaks to d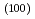 indicated only basal spacing of which interlayer space was equivalent to 2 water layers. Although the
indicated only basal spacing of which interlayer space was equivalent to 2 water layers. Although the  E
E values for I
values for I ions were at similar levels as that for the ionic diffusivity in free water (D
ions were at similar levels as that for the ionic diffusivity in free water (D ) of I
) of I ions at a low dry density of 1.0 Mg/m
ions at a low dry density of 1.0 Mg/m , those increased to 35.24 kJ/mol at a dry density of 1.79 Mg/m
, those increased to 35.24 kJ/mol at a dry density of 1.79 Mg/m . In contrast, the
. In contrast, the  E
E values for Cs
values for Cs ions were clearly higher than that for the D
ions were clearly higher than that for the D of Cs
of Cs ions even at low dry density and further increased to 46.27 kJ/mol at a dry density of 1.79 Mg/m
ions even at low dry density and further increased to 46.27 kJ/mol at a dry density of 1.79 Mg/m . Since it is reported that the activity of water on the surface of montmorillonite is lower than that of free water, such high
. Since it is reported that the activity of water on the surface of montmorillonite is lower than that of free water, such high  E
E for I
for I ions in the interlayer of smectite is considered to be due to the lowering in the activity of interlayer water. On the other hand, Cs
ions in the interlayer of smectite is considered to be due to the lowering in the activity of interlayer water. On the other hand, Cs ions sorb onto smectite by ion exchange with Na
ions sorb onto smectite by ion exchange with Na ions in interlayer. Therefore, such high
ions in interlayer. Therefore, such high  E
E for Cs
for Cs ions in the interlayer of smectite is considered to be due to the combined effects of the ion exchange enthalpy between Cs
ions in the interlayer of smectite is considered to be due to the combined effects of the ion exchange enthalpy between Cs and Na
and Na ions in smectite and the lowering in the activity of interlayer water.
ions in smectite and the lowering in the activity of interlayer water.
Umeki, Hiroyuki
Proceedings of International Workshop on Mobile Fission and Activation Products in Nuclear Waste Disposal, p.11 - 20, 2009/05
no abstracts in English
Iida, Yoshihisa; Yamaguchi, Tetsuji; Tanaka, Tadao; Kitamura, Akira; Nakayama, Shinichi
Proceedings of International Workshop on Mobile Fission and Activation Products in Nuclear Waste Disposal, p.135 - 145, 2009/00
We performed dissolution experiments of iron-selenium compounds from both undersaturation and oversaturation directions to determine the solubility limiting solid of selenium in the presence of iron under anoxic conditions. The solid products was identified to be iron diselenide but the concentration of selenium was 9 orders of magnitude higher than the value calculated from the existing thermodynamic data of FeSe , therefore FeSe
, therefore FeSe was not considered to be the solubility limiting solid. The pH and Eh dependencies of the concentration of selenium were best interpreted as the dissolution reaction of Se(cr), but the concentration of selenium was still 3 orders of magnitude higher than the value calculated from the existing thermodynamic data of Se(cr). Because any selenium compounds other than FeSe
was not considered to be the solubility limiting solid. The pH and Eh dependencies of the concentration of selenium were best interpreted as the dissolution reaction of Se(cr), but the concentration of selenium was still 3 orders of magnitude higher than the value calculated from the existing thermodynamic data of Se(cr). Because any selenium compounds other than FeSe was not detected by XRD and the concentration of selenium was higher than the solubility of Se(cr), the solubility limiting solid was considered to be amorphous selenium.
was not detected by XRD and the concentration of selenium was higher than the solubility of Se(cr), the solubility limiting solid was considered to be amorphous selenium.
Sato, Haruo
Proceedings of International Workshop on Mobile Fission and Activation Products in Nuclear Waste Disposal, p.173 - 184, 2009/00
no abstracts in English